Give Examples of a Property or Use of Three Hydrocarbons
The unsaturated hydrocarbons are of two types. Alkanes and cycloalkanes are examples of saturated hydrocarbon.

Chemical Properties Of Alkanes What Are Examples Of Alkanes Chemical Property Chemistry Basics Chemical
Open chain hydrocarbons are also called aliphatic hydrocarbons or acyclic hydrocarbons.
. Their chemical formulas consist of only carbon and hydrogen atoms in a variety of ratios and chemical configurations. Whereas ethane has only one covalent bond and is a saturated hydrocarbon. We can say that they are the basis of the current economy.
Give at least 3 examples of aromatic compounds as alkane derivatives. Saturated hydrocarbons burn and give a blue non-sooty flame in air. Saturated hydrocarbons have a less amount of carbon atoms bonded to a high number of hydrogen atoms.
Carbon-to-hydrogen ratio of aromatic hydrocarbons is low. Unsaturated hydrocarbons contain at least one carbon - carbon double or triple bond. Unsaturated hydrocarbons burn with a yellow sooty flame.
For the synthesis of drugs dyes and explosives an aryl hydrocarbon known as Phenanthrene is used. It is a highly flammable gas one of those that constitute natural gas and is capable of producing frostbite. Give at least 3 examples of aromatic hydrocarbons with the ortho meta and para systems for the disubstituted compounds.
These are the waxes. In terms of hybridization they have Sp 3 hybridised carbon atom with no Sp 2 or Sp hybridised carbon atoms. Eventually a point is reached at which the materials are solids at room temperature.
The simplest hydrocarbon is methane. Example methane ethane etc. Learn more about aromatic hydrocarbons their properties and uses and see examples.
Ethane C 2 H 6. We can find hydrocarbons in natural gas crude oil coal and plant life. Aromatic hydrocarbons are unsaturated cyclic compounds that are made up primarily of hydrogen and carbon atoms.
Still larger hydrocarbon molecules serve as lubricating oils and greases. Benzene paraffin and methane for example are hydrocarbons. The alkane CH 3 CH 2 388 CH 3 in which 390 carbon atoms are bonded in a continuous chain has been synthesized as an example of a so-called superlong alkane.
They are together called alkanes which have a general formula C n H 2n2. The simplest form of saturated hydrocarbons includes methane CH 4 ethane C 2 H 6 propane C 3 H 8 etc. Ration of Carbon To Hydrogen Atoms.
C Functional group An atomgroup of atoms joined in a specific manner which is responsible for the characteristic chemical properties of the organic compunds. In general the term saturated hydrocarbons is most used to denote acyclic hydrocarbons also known as alkanes. Unsaturated hydrocarbons are composed solely of carbon and hydrogen atoms.
For example CH 4 C 3 H 6. BSaturated hydrocarbons contain carbon- carbon single bonds. Hydrocarbons The alkanes and alkenes are examples of homologous series.
Slightly larger hydrocarbons are known as kerosene or jet fuel diesel fuel and heating oil. Plastic industry and petrochemical industries make use of aromatic hydrocarbons extensively. It is a gas with a repulsive odor very flammable present in the atmosphere of the great gaseous planets and in ours it is the product of the decomposition of organic matter or a product of mining activities.
My mom knows what numbers to use but isnt sure how to explain to me how to. Several thousand carbon atoms are joined together in molecules of hydrocarbon polymers such as polyethylene polypropylene and polystyrene. But they also have numerous uses in other fields.
Some of the examples of this type of compound are- Ethane C 2 H 6 Propane C 3 H 8 Butane C 4 H 10 and Octane C 8 H 18. Alkenes alkynes and aromatic hydrocarbons are examples of unsaturated hydrocarbons. To read more about hydrocarbons their types subtypes examples structure and application in real-life please visit Vedantu.
Compounds like methane butane propane and hexane are all hydrocarbons. Alkynes aromatic and. Methane consists of one carbon atom with four hydrogen atoms stuck to it.
If it has common names give also the common names 3. These types of hydrocarbons dont have double or triple bonds. Saturated hydrocarbons burn with a blue non-sooty flame.
Natural gas and fuels - Many of the natural fuel sources we use are hydrocarbons. Gaseous hydrocarbons are methane and propane liquid hydrocarbons are hexane and benzene low melting solids or waxes hydrocarbons are paraffin wax and naphthalene and polymeric chains of hydrocarbons include polystyrene polypropylene and polyethylene. Explore the definition and examples of unsaturated hydrocarbon and learn about the three types.
There are various types of hydrocarbons depending on how the molecules formed. Classification of Hydrocarbons The compounds containing only carbon and hydrogen in their molecules are called hydrocarbons. We use hydrocarbons as solvents and fuels.
Explain why some proteins are made by nearly all cells and give two examples I put they are needed for basic cell life but I dont know what two examples to put. Aromatic hydrocarbons have a pleasant odor. Which are formed when different carbon atoms join to form an open-chain or a ringed structure.
For example ethene is an alkene that has a double covalent bond and ethylene is an example of alkyne which has a triple covalent bond. Aliphatic hydrocarbons do not have a pleasant odor. Because of the flammability of saturated hydrocarbons that ultimately release a lot of energy saturated hydrocarbons are often used as a fuel source of vehicle and.
Trinitrotoluene or TNT is a very important aromatic hydrocarbon which is widely used for explosive purposes. These single bonded compounds are the simplest hydrocarbons. Carbon makes double or triple bonds with other carbon atoms.
Give 2 examples of mixed numbers that when multiplied by 34 give a product between 34 and 1. In this way we find these components in the plastics insecticides and even cosmetics or soaps. The unsaturated hydrocarbons contain multiple bonds.
A homologous series is a group of chemicals which have similar chemical properties and can be represented by a general formula. Hydrocarbons are classified into two categories known as open chain hydrocarbons and closed chain hydrocarbons. The main applications of hydrocarbons are given in transport as fuel and in industry.
Carbon-to-hydrogen ratio of aliphatic hydrocarbons is high. They contain only sp 3 hybridized carbon atoms and their general formula is C n H 2n2.

Alkanes Definition Nomenclature Preparation Properties Geeksforgeeks

Three States Of Matter Definition Classification Videos Examples States Of Matter 3 States Of Matter Structure Of Matter
Ch105 Chapter 7 Alkanes And Halogenated Hydrocarbons Chemistry
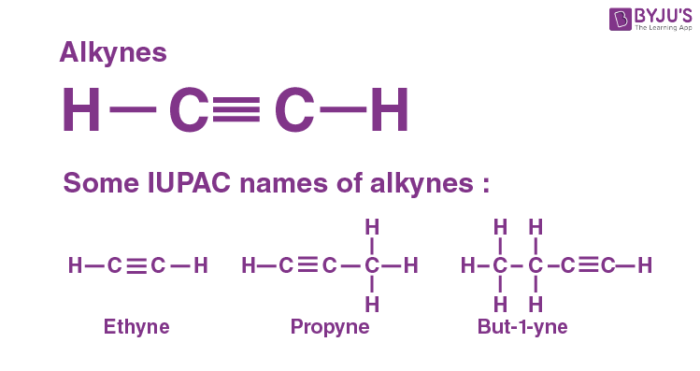
Alkynes Preparation Properties Structure Examples With Videos
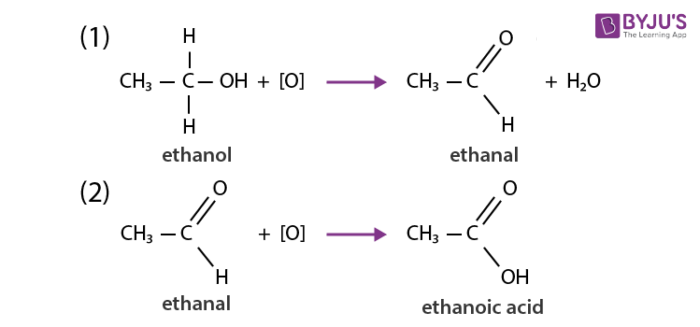
Chemical Properties Of Carbon Oxidation Reaction Addition Reaction Substitution Reactions Combustion Reaction Of Carbon And Its Compounds With Faqs On Chemical Properties Of Carbon

Alkanes Formula List Structure Definition Examples Videos

Heat Of Fusion And Melting Point Crystalline Vs Amorphous Solids The Crystalline Solid Is Made Up Of The Unit Cells Melting Point Unit Cell Crystalline Solid

General Introduction To Organic Compounds Properties Uses With Videos

Physical Chemical Properties Of Alkanes With Examples Videos
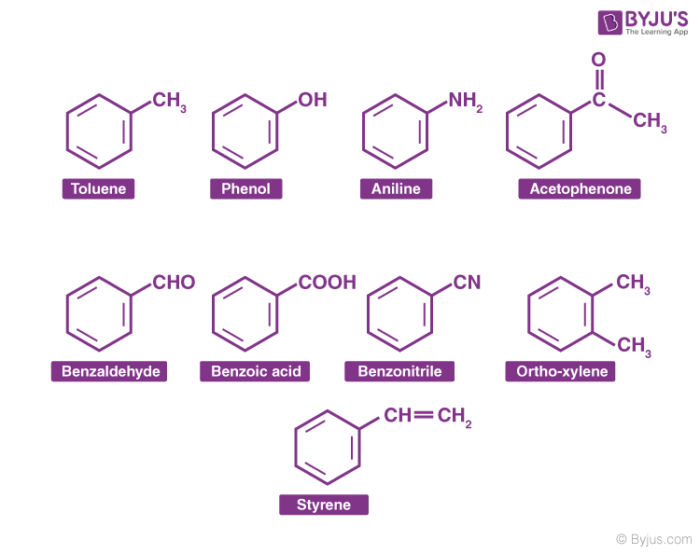
Aromatic Compounds Definition Example Properties Nomenclature With Videos

Unsaturated Hydrocarbon Definition Examples And Uses

Structure Of Glycosidic Bond Biochemistry Notes Teaching Chemistry Biochemistry

Selina Concise Chemistry Class 10 Icse Solutions Organic Chemistry Organic Chemistry Chemistry Class Acetic Anhydride
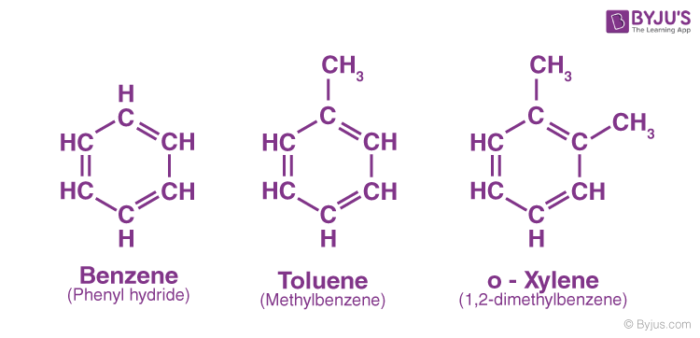
Aromatic Hydrocarbons Definition Examples Properties Uses Of Aromatic Hydrocarbons

Ch105 Chapter 7 Alkanes And Halogenated Hydrocarbons Chemistry

Difference Between Crystalline And Amorphous Solids Http Www Curlyarrows Com Difference Crystalline Amo 11th Chemistry How To Memorize Things Physics Courses
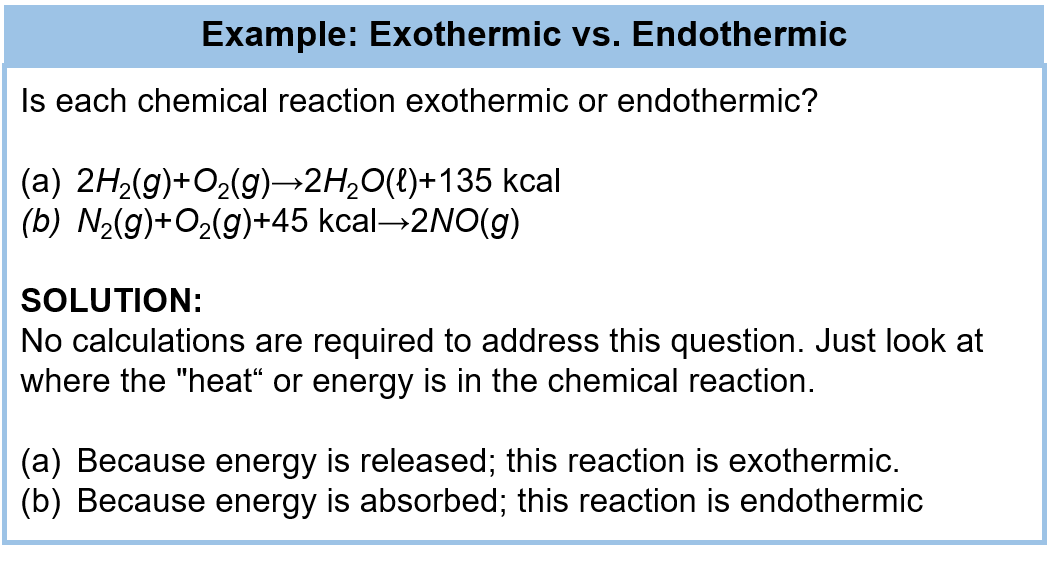
Ch105 Chapter 7 Alkanes And Halogenated Hydrocarbons Chemistry

Comments
Post a Comment